Decoding The PH Chart: A Complete Information To Acids, Bases, And The PH Scale
Decoding the pH Chart: A Complete Information to Acids, Bases, and the pH Scale
Associated Articles: Decoding the pH Chart: A Complete Information to Acids, Bases, and the pH Scale
Introduction
With nice pleasure, we’ll discover the intriguing subject associated to Decoding the pH Chart: A Complete Information to Acids, Bases, and the pH Scale. Let’s weave attention-grabbing info and provide contemporary views to the readers.
Desk of Content material
Decoding the pH Chart: A Complete Information to Acids, Bases, and the pH Scale
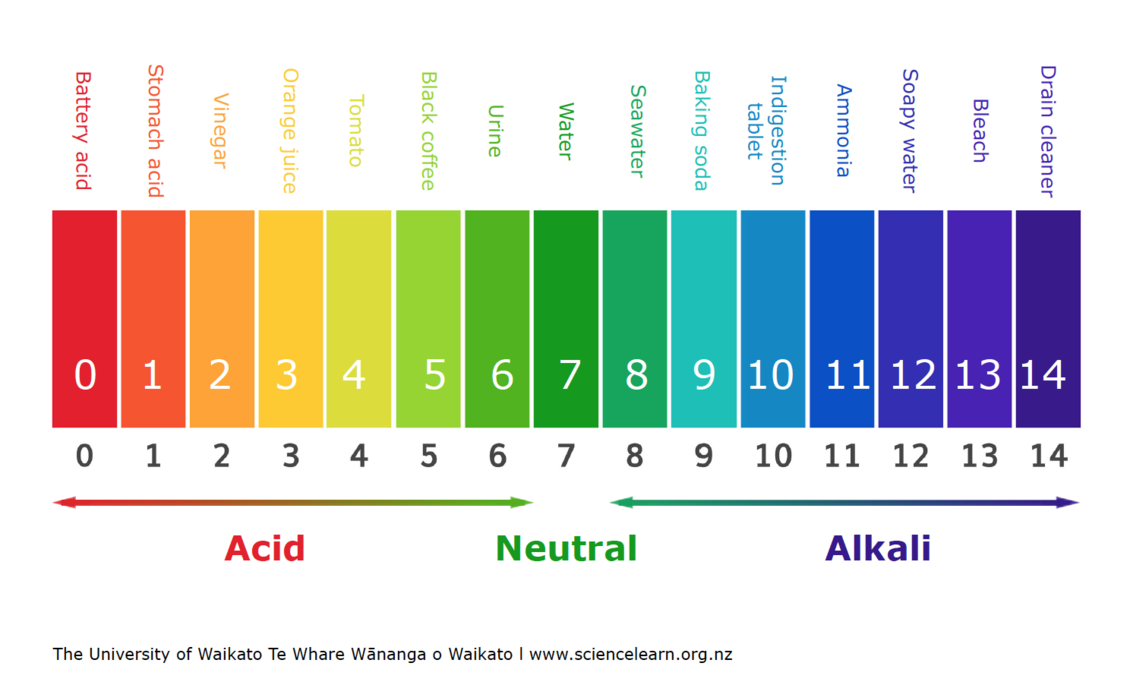
The pH scale is a elementary idea in chemistry, offering a quantitative measure of the acidity or basicity (alkalinity) of an answer. Understanding the pH chart and its implications is essential in numerous fields, from environmental science and agriculture to medication and industrial processes. This text delves into the intricacies of the pH scale, explaining its workings, significance, and purposes.
Understanding the pH Scale:
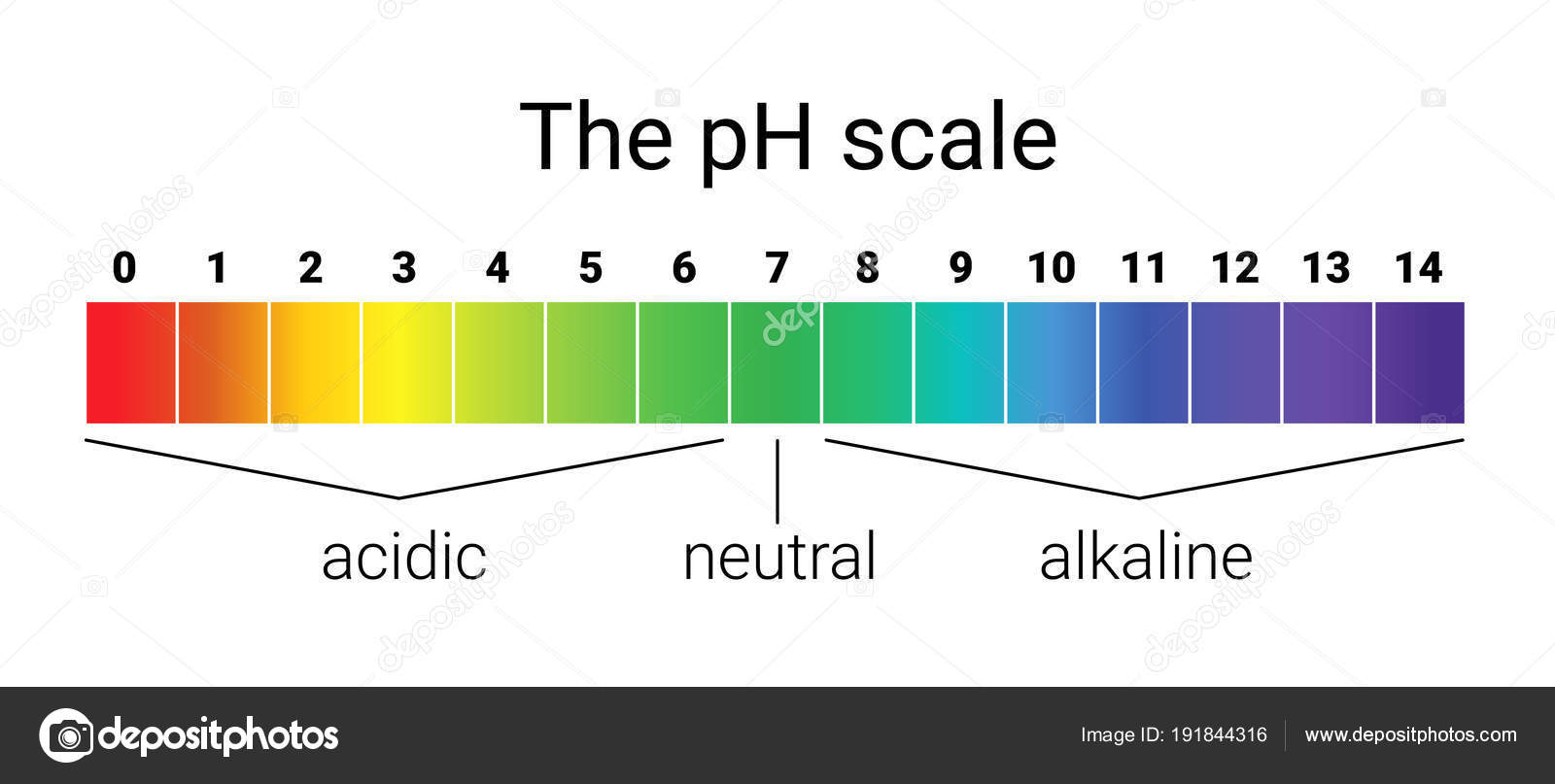
The pH scale is a logarithmic scale starting from 0 to 14, with 7 representing neutrality. A pH worth under 7 signifies acidity, whereas a worth above 7 signifies basicity or alkalinity. Every complete quantity change on the size represents a tenfold change within the focus of hydrogen ions (H⁺) within the answer. For instance, an answer with a pH of three is ten instances extra acidic than an answer with a pH of 4, and 100 instances extra acidic than an answer with a pH of 5.
The size relies on the focus of hydrogen ions (H⁺) in an answer. These ions are fashioned when acids dissociate in water. The pH is outlined because the detrimental logarithm (base 10) of the hydrogen ion focus:
pH = -log₁₀[H⁺]
The place [H⁺] represents the focus of hydrogen ions in moles per liter (mol/L).
Conversely, the focus of hydroxide ions (OH⁻), that are attribute of bases, could be calculated utilizing the next relationship:
pOH = -log₁₀[OH⁻]
The sum of pH and pOH for any aqueous answer at 25°C is at all times 14:
pH + pOH = 14
Acids and their pH:
Acids are substances that donate hydrogen ions (protons, H⁺) when dissolved in water. They enhance the focus of H⁺ ions, resulting in a decrease pH. Acids could be labeled as robust or weak relying on their diploma of dissociation in water.
-
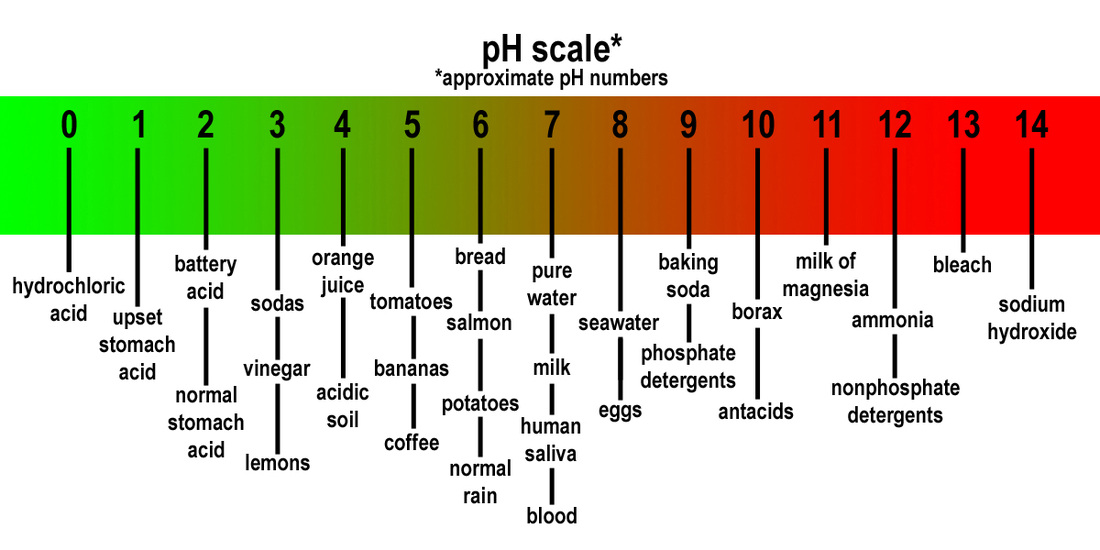
Robust acids: These acids fully dissociate in water, releasing all their hydrogen ions. Examples embrace hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃). These acids have a pH near 0.
-
Weak acids: These acids solely partially dissociate in water, releasing solely a fraction of their hydrogen ions. Examples embrace acetic acid (CH₃COOH), carbonic acid (H₂CO₃), and citric acid (C₆H₈O₇). Their pH values are usually between 3 and 6.
The power of an acid just isn’t immediately associated to its focus. A dilute answer of a powerful acid can nonetheless have a decrease pH than a concentrated answer of a weak acid.
Bases and their pH:
Bases are substances that settle for hydrogen ions (protons, H⁺) or donate hydroxide ions (OH⁻) when dissolved in water. They enhance the focus of OH⁻ ions, resulting in the next pH. Just like acids, bases will also be labeled as robust or weak.
-
Robust bases: These bases fully dissociate in water, releasing all their hydroxide ions. Examples embrace sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)₂). These bases have a pH near 14.
-
Weak bases: These bases solely partially dissociate in water, releasing solely a fraction of their hydroxide ions. Examples embrace ammonia (NH₃) and plenty of natural amines. Their pH values are usually between 8 and 11.
The Significance of pH:
The pH of an answer has profound implications throughout quite a few fields:
-
Biology and Medication: The pH of bodily fluids is tightly regulated. For example, blood pH wants to stay inside a slender vary (7.35-7.45) for correct physiological operate. Deviations from this vary can result in acidosis (low pH) or alkalosis (excessive pH), each of which could be life-threatening. Many enzymes and proteins operate optimally inside particular pH ranges. Moreover, pH performs an important position in drug absorption and efficacy.
-
Environmental Science: The pH of soil and water considerably influences the supply of vitamins to vegetation and the survival of aquatic organisms. Acid rain, attributable to atmospheric air pollution, can drastically decrease the pH of lakes and rivers, harming aquatic life. Monitoring soil pH is essential for efficient agriculture, as completely different vegetation thrive at completely different pH ranges.
-
Industrial Processes: Many industrial processes are extremely delicate to pH. For instance, the manufacturing of prescribed drugs, meals processing, and wastewater therapy all require cautious pH management. pH indicators and meters are important instruments in these industries for monitoring and adjusting pH ranges.
-
Meals and Beverage Trade: The pH of meals merchandise impacts their style, texture, and shelf life. For instance, the fermentation course of in yogurt and cheese manufacturing depends on exact pH management. The pH of drinks additionally impacts their taste and preservation.
Measuring pH:
A number of strategies are used to measure the pH of an answer:
-
pH indicators: These are substances that change coloration relying on the pH of the answer. Litmus paper, a standard pH indicator, turns crimson in acidic options and blue in fundamental options. Extra refined indicators present a wider vary of coloration modifications throughout the pH scale.
-
pH meters: These digital gadgets present a extra exact measurement of pH. They encompass a pH-sensitive electrode that’s immersed within the answer, and a meter that shows the pH worth. pH meters are generally utilized in laboratories and industrial settings.
Buffers and pH Regulation:
Buffers are options that resist modifications in pH upon the addition of small quantities of acid or base. They encompass a weak acid and its conjugate base, or a weak base and its conjugate acid. Buffers play an important position in sustaining the pH of organic methods, reminiscent of blood, which incorporates a bicarbonate buffer system.
Purposes of pH Chart and its Understanding:
The understanding of the pH chart and its implications extends to quite a few sensible purposes:
-
Agriculture: Soil testing to find out pH ranges is important for optimizing crop yields. Adjusting soil pH via the addition of lime (for acidic soils) or sulfur (for alkaline soils) is a standard observe.
-
Water Remedy: Monitoring and adjusting the pH of water is essential for making certain its potability and stopping corrosion in pipes.
-
Wastewater Remedy: pH management is important in wastewater therapy vegetation to optimize the effectivity of varied processes, reminiscent of coagulation and flocculation.
-
Swimming Pool Upkeep: Sustaining the correct pH stage in swimming swimming pools is essential for stopping eye irritation and defending pool tools.
-
Meals Preservation: Controlling the pH of meals merchandise can inhibit the expansion of microorganisms and lengthen their shelf life.
-
Cosmetics and Private Care Merchandise: The pH of skincare merchandise is fastidiously formulated to be appropriate with the pores and skin’s pure pH.
Conclusion:
The pH chart and the understanding of acids and bases are elementary ideas with far-reaching purposes throughout numerous scientific disciplines and industries. From sustaining the well being of our our bodies and the atmosphere to optimizing industrial processes and making certain meals security, the power to measure and management pH is important for quite a few facets of contemporary life. The continued growth of superior methods for pH measurement and management will undoubtedly result in additional developments in these fields. This complete overview underscores the significance of mastering the pH scale and its related ideas for anybody concerned in scientific analysis, industrial processes, or environmental administration. The intricate relationship between pH, acids, and bases continues to be a cornerstone of chemical understanding and sensible utility.



Closure
Thus, we hope this text has offered worthwhile insights into Decoding the pH Chart: A Complete Information to Acids, Bases, and the pH Scale. We hope you discover this text informative and useful. See you in our subsequent article!