Mastering The Solubility Chart: A Complete Information For AP Chemistry College students
Mastering the Solubility Chart: A Complete Information for AP Chemistry College students
Associated Articles: Mastering the Solubility Chart: A Complete Information for AP Chemistry College students
Introduction
On this auspicious event, we’re delighted to delve into the intriguing matter associated to Mastering the Solubility Chart: A Complete Information for AP Chemistry College students. Let’s weave attention-grabbing info and provide contemporary views to the readers.
Desk of Content material
Mastering the Solubility Chart: A Complete Information for AP Chemistry College students

The solubility chart is an indispensable device in AP Chemistry, offering a concise but highly effective abstract of the solubility of assorted ionic compounds in water. Understanding and successfully using this chart is essential for predicting response outcomes, balancing equations, and comprehending the rules of equilibrium and precipitation reactions. This text offers a complete overview of solubility charts, their interpretation, widespread exceptions, and their software in fixing AP-level chemistry issues.
Understanding Solubility:
Solubility refers back to the most quantity of a solute that may dissolve in a given quantity of solvent at a particular temperature and strain. For ionic compounds in water, solubility is set by the steadiness between the enticing forces between the ions within the crystal lattice and the enticing forces between the ions and water molecules (hydration). If the hydration vitality is larger than the lattice vitality, the compound might be soluble; if the lattice vitality is larger, the compound might be insoluble or sparingly soluble.
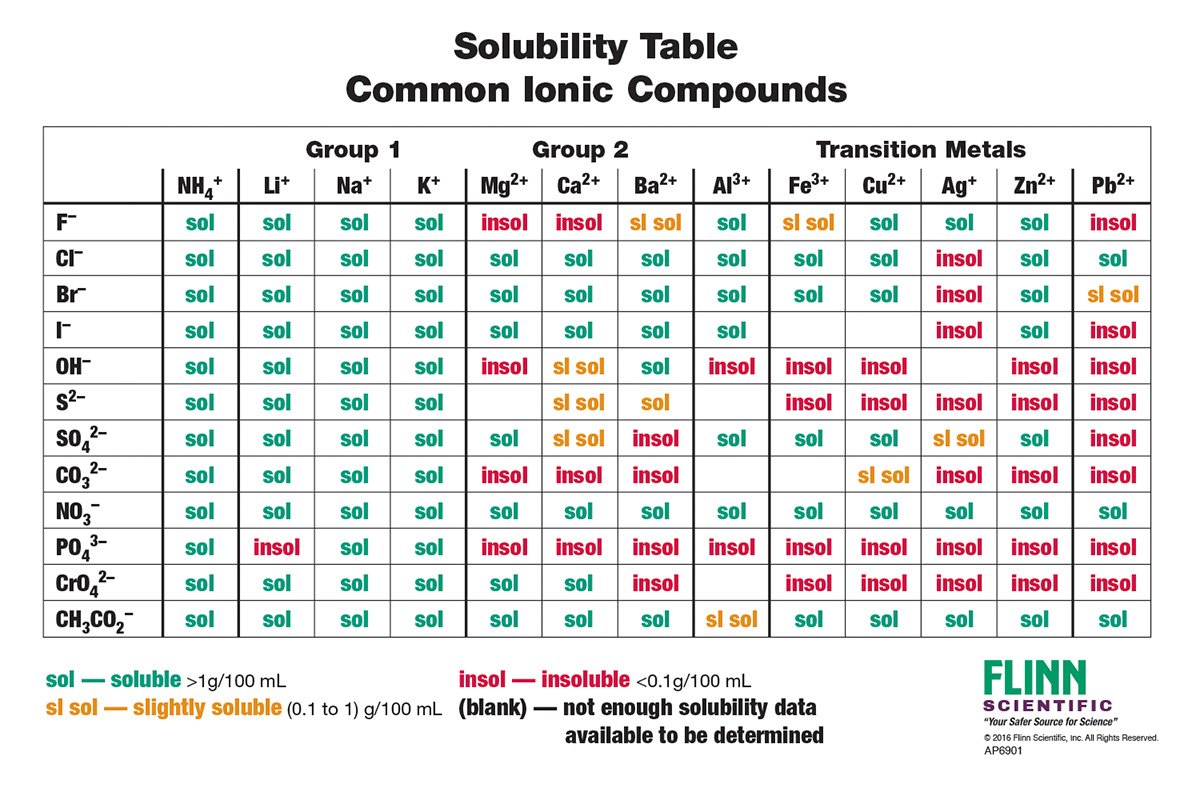
Construction and Interpretation of the Solubility Chart:
Solubility charts usually current info in a desk format. The chart’s rows signify the anions (negatively charged ions), and the columns signify the cations (positively charged ions). The intersection of a row and column signifies the solubility of the ionic compound fashioned by that particular cation and anion. Solubility is usually represented qualitatively utilizing abbreviations resembling:
- S: Soluble (typically thought of to dissolve to at the least 0.1 M)
- SS: Barely Soluble (solubility between 0.01 M and 0.1 M)
- I: Insoluble (solubility lower than 0.01 M)
- D: Decomposes (reacts with water, producing a unique compound)
Some charts may use a extra nuanced scale with extra classes or numerical solubility values. Nonetheless, the essential qualitative classification (S, SS, I) is adequate for many AP Chemistry functions.
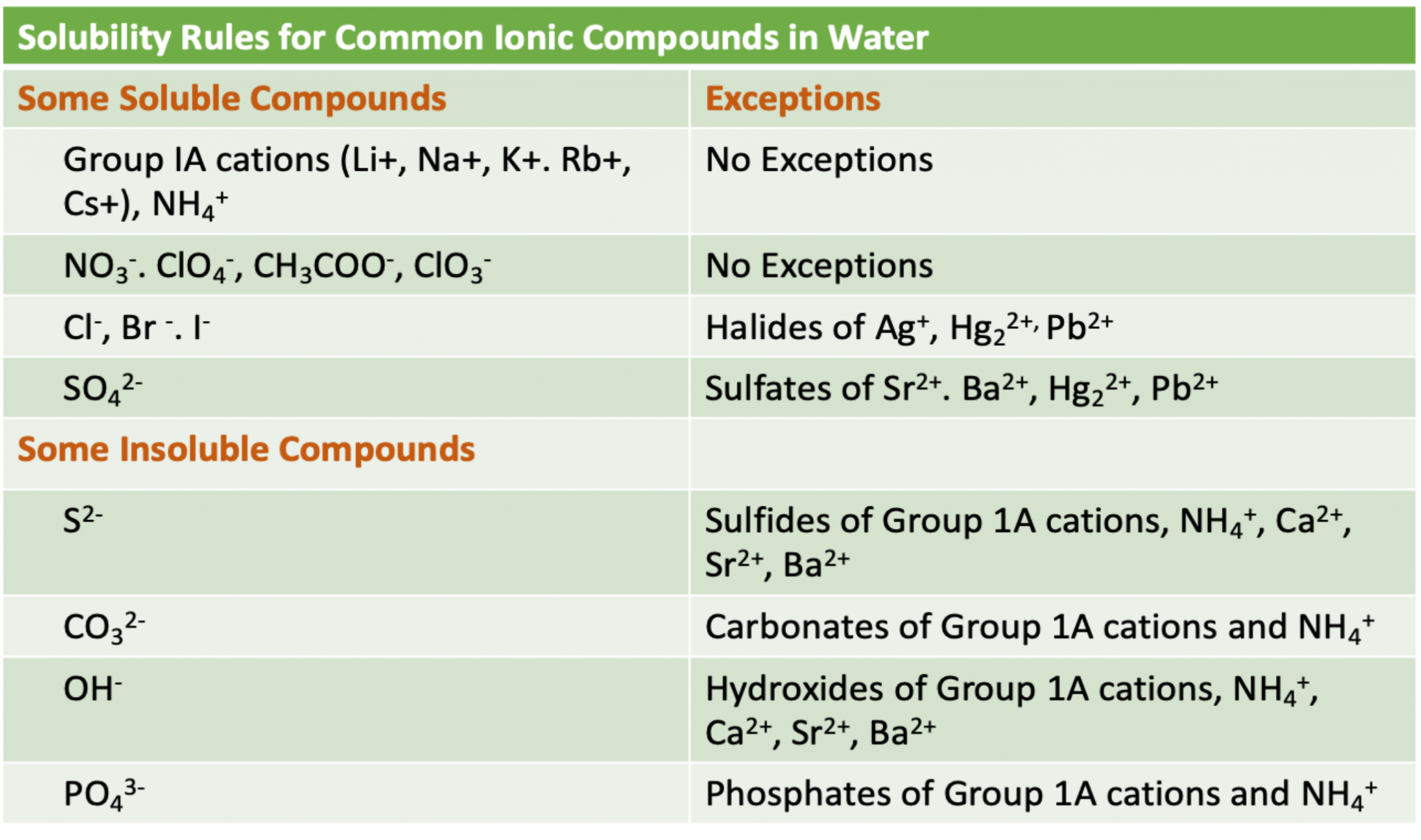
Key Solubility Guidelines:
Whereas solubility charts present a fast reference, understanding the underlying solubility guidelines is important for deciphering the chart and predicting solubility with out immediately consulting it. These guidelines are generalizations, and exceptions exist, emphasizing the significance of utilizing the chart to substantiate predictions. The commonest solubility guidelines embody:
-
Group 1A (alkali metals) and ammonium (NH₄⁺) salts: Usually soluble. These cations type robust bonds with water molecules, overcoming the lattice vitality.
-
Nitrate (NO₃⁻), acetate (CH₃COO⁻), perchlorate (ClO₄⁻), and chlorate (ClO₃⁻) salts: Usually soluble. These anions are comparatively giant and have low cost density, resulting in weaker lattice energies.
-
Halide salts (Cl⁻, Br⁻, I⁻): Usually soluble, apart from these fashioned with silver (Ag⁺), lead (Pb²⁺), and mercury(I) (Hg₂²⁺). This exception highlights the robust lattice energies fashioned by these particular cation-anion mixtures.
-
Sulfate (SO₄²⁻) salts: Usually soluble, apart from these fashioned with calcium (Ca²⁺), strontium (Sr²⁺), barium (Ba²⁺), lead (Pb²⁺), and mercury(I) (Hg₂²⁺). Just like halide exceptions, these insoluble sulfates replicate robust lattice energies.
-
Hydroxide (OH⁻) salts: Usually insoluble, apart from these fashioned with Group 1A metals and barium (Ba²⁺). Hydroxides are inclined to have excessive lattice energies, making them largely insoluble.
-
Sulfide (S²⁻), carbonate (CO₃²⁻), phosphate (PO₄³⁻), chromate (CrO₄²⁻), and oxalate (C₂O₄²⁻) salts: Usually insoluble, apart from these fashioned with Group 1A metals and ammonium (NH₄⁺). These polyatomic anions usually type salts with robust lattice energies.
Frequent Exceptions and Concerns:
It is essential to keep in mind that these guidelines are generalizations. Many exceptions exist, and the solubility chart ought to at all times be consulted for definitive info. Elements influencing solubility past the cation and anion identification embody:
- Temperature: Solubility usually will increase with temperature, although there are exceptions.
- Frequent Ion Impact: The presence of a standard ion in resolution reduces the solubility of a sparingly soluble salt.
- Complicated Ion Formation: The formation of advanced ions can considerably improve the solubility of a usually insoluble salt.
- pH: The pH of the answer can have an effect on the solubility of salts containing weak acids or bases.
Purposes of the Solubility Chart in AP Chemistry:
The solubility chart performs an important function in a number of key areas of AP Chemistry:
-
Predicting Precipitation Reactions: When two options containing soluble salts are blended, a precipitate (insoluble stable) might type if the mixture of cations and anions produces an insoluble compound, as indicated by the solubility chart.
-
Writing Internet Ionic Equations: The solubility chart helps determine spectator ions (ions that don’t take part within the response) and permits you to write concise web ionic equations representing solely the species immediately concerned within the precipitation.
-
Qualitative Evaluation: Solubility charts are important in qualitative evaluation, the place the aim is to determine the presence of particular ions in an answer by selective precipitation reactions.
-
Equilibrium Calculations: Solubility charts present preliminary info for equilibrium calculations involving sparingly soluble salts, permitting the willpower of solubility product constants (Ksp) and understanding the components affecting solubility.
-
Understanding Buffer Programs: Solubility will be influenced by pH, a vital think about buffer programs. The solubility chart helps predict the habits of sparingly soluble salts in options with various pH.
Instance Drawback:
Take into account mixing options of silver nitrate (AgNO₃) and sodium chloride (NaCl). Utilizing the solubility chart:
- Establish the doable merchandise: AgCl and NaNO₃.
- Verify solubility: AgNO₃ and NaCl are soluble (S). NaNO₃ is soluble (S). AgCl is insoluble (I).
- Write the balanced molecular equation: AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
- Write the whole ionic equation: Ag⁺(aq) + NO₃⁻(aq) + Na⁺(aq) + Cl⁻(aq) → AgCl(s) + Na⁺(aq) + NO₃⁻(aq)
- Write the online ionic equation: Ag⁺(aq) + Cl⁻(aq) → AgCl(s)
This instance demonstrates how the solubility chart is used to foretell the formation of a precipitate and write acceptable equations.
Conclusion:
The solubility chart is a elementary device for fulfillment in AP Chemistry. Mastering its interpretation and understanding the underlying rules of solubility are important for tackling advanced issues associated to precipitation reactions, equilibrium, and qualitative evaluation. By combining data of solubility guidelines with cautious statement of the chart, college students can confidently predict response outcomes and acquire a deeper understanding of the habits of ionic compounds in aqueous options. Common apply and problem-solving utilizing the solubility chart are essential for growing proficiency and attaining success within the AP Chemistry examination.






Closure
Thus, we hope this text has supplied useful insights into Mastering the Solubility Chart: A Complete Information for AP Chemistry College students. We admire your consideration to our article. See you in our subsequent article!