Unveiling The Reactive Nature Of Components: A Complete Look At The Valency Chart Of The First 30 Components
Unveiling the Reactive Nature of Components: A Complete Take a look at the Valency Chart of the First 30 Components
Associated Articles: Unveiling the Reactive Nature of Components: A Complete Take a look at the Valency Chart of the First 30 Components
Introduction
With nice pleasure, we’ll discover the intriguing matter associated to Unveiling the Reactive Nature of Components: A Complete Take a look at the Valency Chart of the First 30 Components. Let’s weave attention-grabbing info and provide recent views to the readers.
Desk of Content material
Unveiling the Reactive Nature of Components: A Complete Take a look at the Valency Chart of the First 30 Components

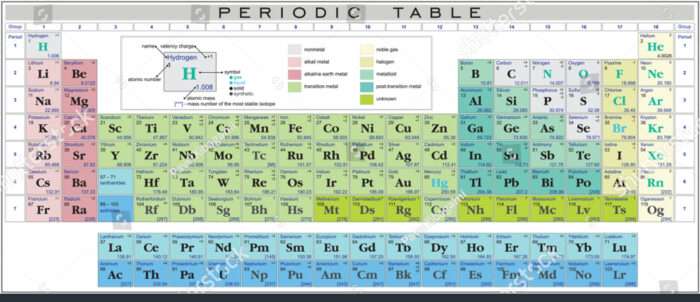
The periodic desk, a cornerstone of chemistry, organizes parts primarily based on their atomic construction and recurring chemical properties. One of the crucial essential facets figuring out a component’s conduct is its valency – the variety of electrons an atom wants to realize, lose, or share to realize a steady electron configuration, usually resembling that of a noble fuel. Understanding valency is key to predicting how parts will react and type compounds. This text delves into the valency chart of the primary 30 parts, offering an in depth clarification of their valencies and the underlying rules governing them.
Understanding Valency: A Basis for Chemical Bonding
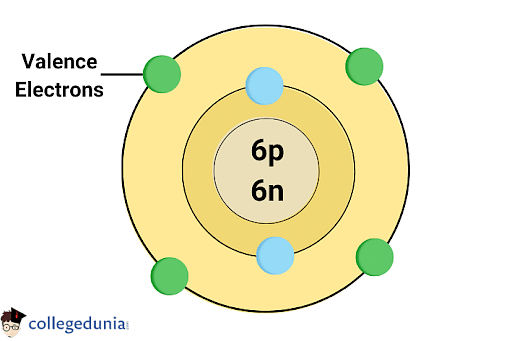
Valency is intimately linked to an atom’s electron configuration, particularly the electrons in its outermost shell, often known as valence electrons. Atoms try for stability, usually reaching it by buying a full outermost shell, usually resembling the electron configuration of a noble fuel (Group 18 parts). This drive for stability dictates how atoms work together, forming chemical bonds.
There are three main methods atoms obtain steady configurations:
-
Ionic Bonding: Atoms switch electrons, ensuing within the formation of ions – positively charged cations (fashioned by electron loss) and negatively charged anions (fashioned by electron acquire). The electrostatic attraction between these oppositely charged ions constitutes the ionic bond. Components with low ionization energies (simply lose electrons) usually type cations, whereas parts with excessive electron affinities (simply acquire electrons) type anions.
-
Covalent Bonding: Atoms share electrons to realize a steady electron configuration. This sharing creates a covalent bond, the place the shared electrons are drawn to the nuclei of each atoms. The sort of bonding is prevalent amongst nonmetals.
-
Metallic Bonding: In metals, valence electrons are delocalized, forming a "sea" of electrons which might be shared amongst many metallic atoms. This delocalization accounts for the attribute properties of metals, resembling electrical and thermal conductivity, malleability, and ductility.
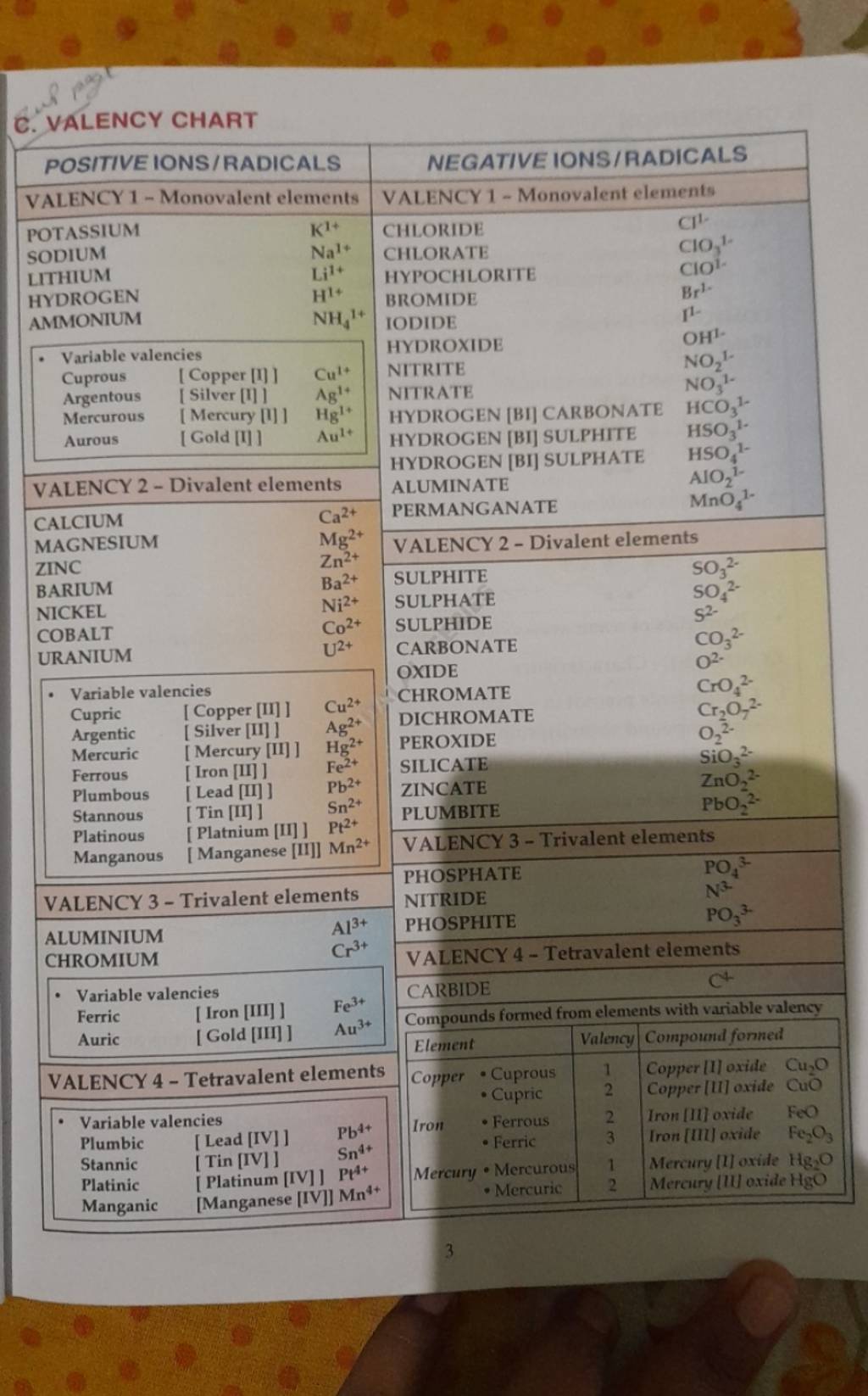
The Valency Chart of the First 30 Components: A Detailed Evaluation
The next desk presents the valency chart for the primary 30 parts, together with explanations for variations and exceptions:
| Ingredient | Image | Atomic Quantity | Electron Configuration | Valency | Clarification |
|---|---|---|---|---|---|
| Hydrogen | H | 1 | 1s¹ | +1 or -1 | Can lose one electron to turn out to be H⁺ or acquire one electron to turn out to be H⁻ (hydride ion). |
| Helium | He | 2 | 1s² | 0 | Noble fuel; steady electron configuration; inert. |
| Lithium | Li | 3 | 1s²2s¹ | +1 | Loses one electron to realize a steady configuration like He. |
| Beryllium | Be | 4 | 1s²2s² | +2 | Loses two electrons to realize a steady configuration. |
| Boron | B | 5 | 1s²2s²2p¹ | +3 | Loses three electrons or shares three electrons to realize a steady configuration. |
| Carbon | C | 6 | 1s²2s²2p² | +4 or -4 | Can lose 4 electrons or acquire 4 electrons or share 4 electrons to realize a steady configuration. Exhibits variable valency in natural compounds. |
| Nitrogen | N | 7 | 1s²2s²2p³ | -3 or +3 or +5 | Usually positive aspects three electrons to type N³⁻ (nitride ion) or shares electrons to type numerous compounds with variable oxidation states. |
| Oxygen | O | 8 | 1s²2s²2p⁴ | -2 | Beneficial properties two electrons to type O²⁻ (oxide ion). |
| Fluorine | F | 9 | 1s²2s²2p⁵ | -1 | Beneficial properties one electron to type F⁻ (fluoride ion). |
| Neon | Ne | 10 | 1s²2s²2p⁶ | 0 | Noble fuel; steady electron configuration; inert. |
| Sodium | Na | 11 | 1s²2s²2p⁶3s¹ | +1 | Loses one electron to realize a steady configuration like Ne. |
| Magnesium | Mg | 12 | 1s²2s²2p⁶3s² | +2 | Loses two electrons to realize a steady configuration. |
| Aluminum | Al | 13 | 1s²2s²2p⁶3s²3p¹ | +3 | Loses three electrons to realize a steady configuration. |
| Silicon | Si | 14 | 1s²2s²2p⁶3s²3p² | +4 | Shares 4 electrons or kinds covalent bonds. |
| Phosphorus | P | 15 | 1s²2s²2p⁶3s²3p³ | -3 or +3 or +5 | Can acquire three electrons to type P³⁻ (phosphide ion) or share electrons to type numerous compounds with variable oxidation states. |
| Sulfur | S | 16 | 1s²2s²2p⁶3s²3p⁴ | -2 or +4 or +6 | Beneficial properties two electrons to type S²⁻ (sulfide ion) or shares electrons to type numerous compounds with variable oxidation states. |
| Chlorine | Cl | 17 | 1s²2s²2p⁶3s²3p⁵ | -1 | Beneficial properties one electron to type Cl⁻ (chloride ion). |
| Argon | Ar | 18 | 1s²2s²2p⁶3s²3p⁶ | 0 | Noble fuel; steady electron configuration; inert. |
| Potassium | Okay | 19 | 1s²2s²2p⁶3s²3p⁶4s¹ | +1 | Loses one electron to realize a steady configuration like Ar. |
| Calcium | Ca | 20 | 1s²2s²2p⁶3s²3p⁶4s² | +2 | Loses two electrons to realize a steady configuration. |
| Scandium | Sc | 21 | 1s²2s²2p⁶3s²3p⁶4s²3d¹ | +3 | Loses three electrons (4s² and 3d¹), although variable valency is feasible. |
| Titanium | Ti | 22 | 1s²2s²2p⁶3s²3p⁶4s²3d² | +2, +3, +4 | Exhibits variable valency resulting from involvement of 3d electrons. |
| Vanadium | V | 23 | 1s²2s²2p⁶3s²3p⁶4s²3d³ | +2, +3, +4, +5 | Exhibits variable valency resulting from involvement of 3d electrons. |
| Chromium | Cr | 24 | 1s²2s²2p⁶3s²3p⁶4s¹3d⁵ | +2, +3, +6 | Distinctive configuration; variable valency resulting from involvement of 3d electrons. |
| Manganese | Mn | 25 | 1s²2s²2p⁶3s²3p⁶4s²3d⁵ | +2, +3, +4, +6, +7 | Exhibits a variety of valencies resulting from involvement of 3d electrons. |
| Iron | Fe | 26 | 1s²2s²2p⁶3s²3p⁶4s²3d⁶ | +2, +3 | Frequent valencies; 3d electrons take part in bonding. |
| Cobalt | Co | 27 | 1s²2s²2p⁶3s²3p⁶4s²3d⁷ | +2, +3 | Frequent valencies; 3d electrons take part in bonding. |
| Nickel | Ni | 28 | 1s²2s²2p⁶3s²3p⁶4s²3d⁸ | +2 | Predominantly +2 valency; 3d electrons take part in bonding. |
| Copper | Cu | 29 | 1s²2s²2p⁶3s²3p⁶4s¹3d¹⁰ | +1, +2 | Distinctive configuration; variable valency resulting from involvement of 3d electrons. |
| Zinc | Zn | 30 | 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰ | +2 | Loses two 4s electrons; 3d electrons will not be usually concerned in bonding. |
Explaining Variations and Exceptions:
The valency chart reveals some attention-grabbing patterns and exceptions:
-
Noble Gases (He, Ne, Ar): These parts have a full outermost electron shell, rendering them chemically inert and possessing a valency of 0.
-
Transition Metals (Sc to Zn): These parts exhibit variable valencies as a result of involvement of each s and d electrons in bonding. The power distinction between these electron shells is comparatively small, permitting for various numbers of electrons to take part in chemical bonding. This explains the various oxidation states displayed by transition metals. Chromium and copper are notable examples of exceptions of their electron configuration, resulting in their variable valencies.
-
Group 14 Components (C, Si): Carbon, particularly, shows outstanding versatility, forming an unlimited array of natural compounds with various valencies resulting from its means to type a number of covalent bonds.
-
Group 15 and 16 Components (N, P, O, S): These parts can exhibit each destructive and optimistic valencies relying on the electronegativity of the atoms they bond with. They usually take part in covalent bonding, forming compounds with variable oxidation states.
Conclusion:
The valency chart of the primary 30 parts gives an important perception into the reactivity and bonding conduct of those elementary constructing blocks of matter. Whereas the octet rule (reaching eight valence electrons) serves as a helpful guideline, exceptions and variations exist, significantly amongst transition metals. Understanding the underlying rules of electron configuration and the drive for stability permits us to foretell and interpret the chemical conduct of parts and the compounds they type. This information kinds the bedrock of quite a few functions in chemistry, supplies science, and different associated fields, facilitating the design and synthesis of latest supplies with tailor-made properties. Additional exploration into the periodic desk and the intricacies of chemical bonding will reveal much more fascinating nuances within the reactive nature of parts past the primary 30.





Closure
Thus, we hope this text has supplied priceless insights into Unveiling the Reactive Nature of Components: A Complete Take a look at the Valency Chart of the First 30 Components. We hope you discover this text informative and helpful. See you in our subsequent article!